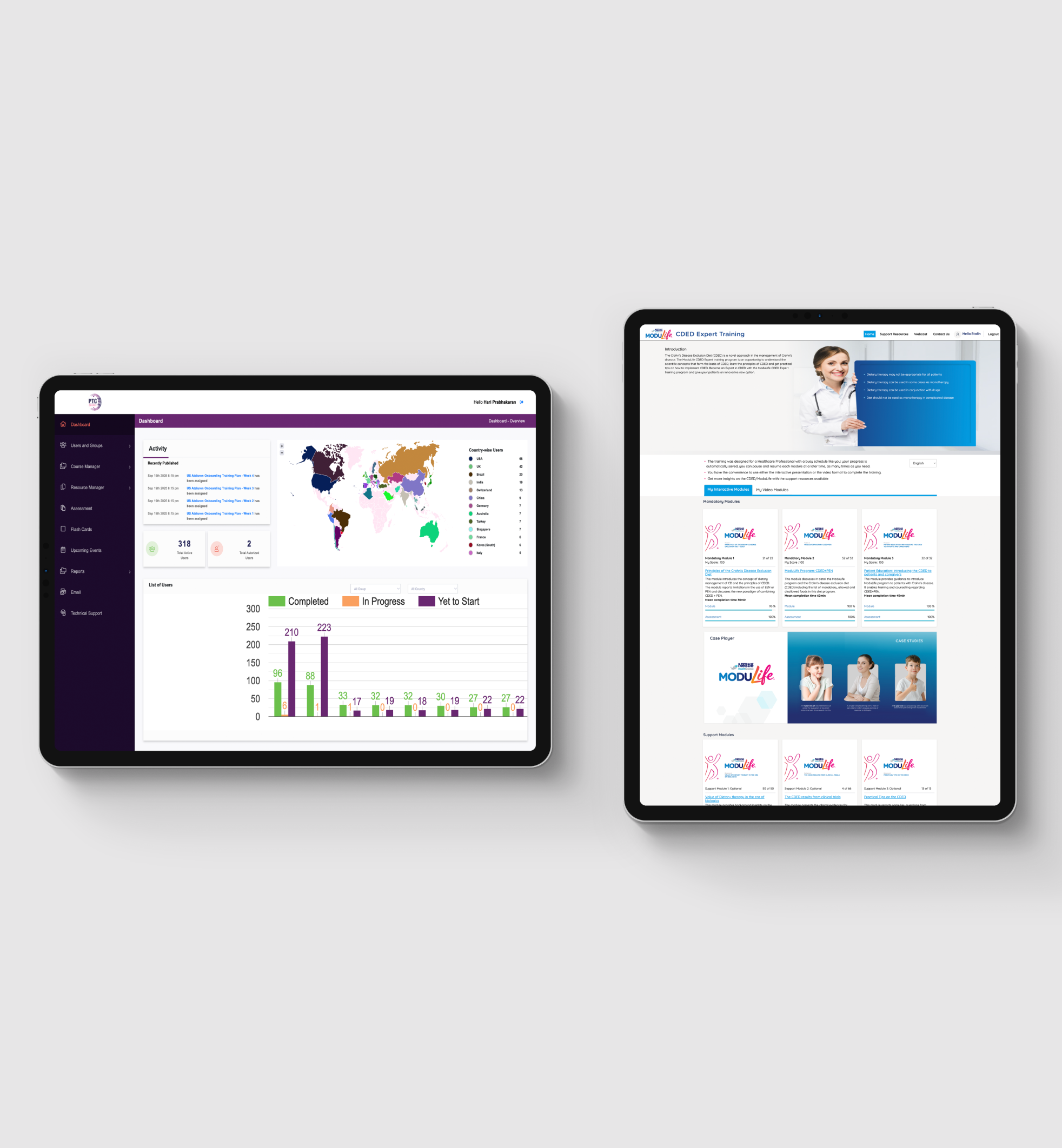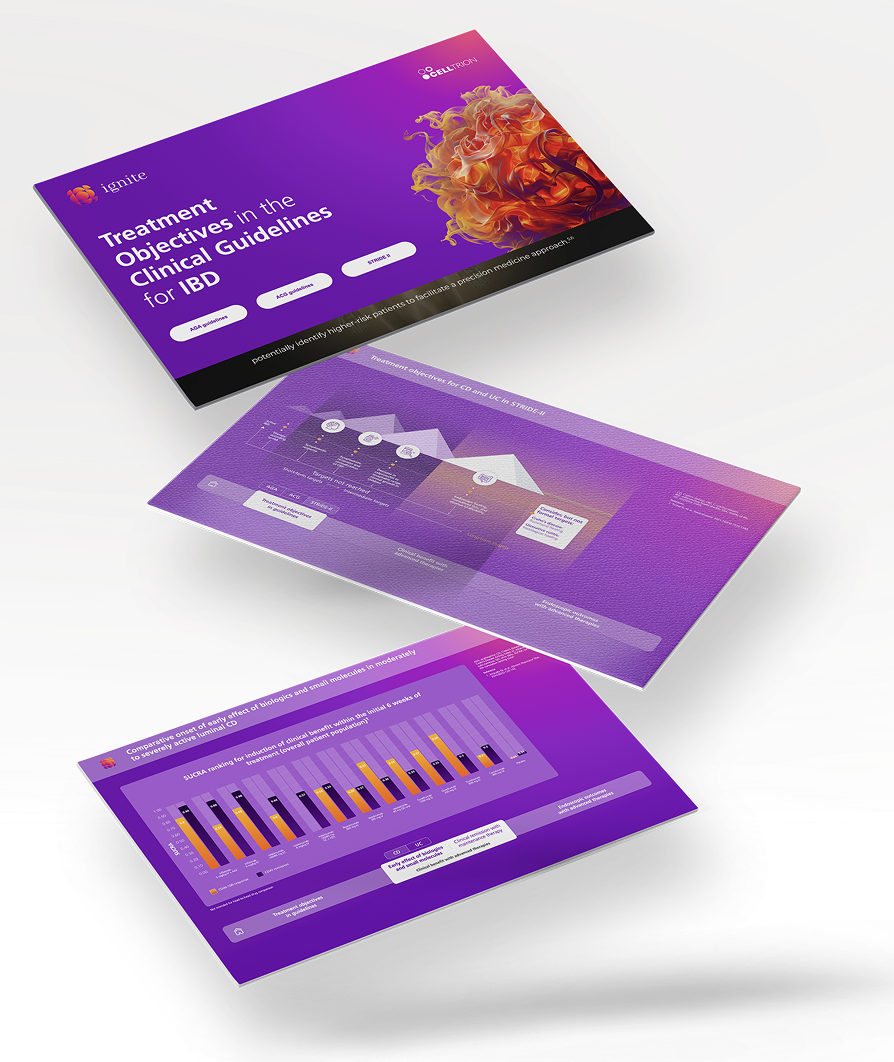
Clinical Evidence Communication
- Scientific Content Development
- Systematic literature reviews and evidence synthesis
- Meta-analyses and health economic analyses
- Development of health technology assessment (HTA) dossiers
- Generation of clinical evidence tables and comparative efficacy summaries
- Publication strategy development and execution planning
Publication Extenders: The Digital Amplification Suite
MedTrix offers a suite of Publication Extenders with Enhanced Content or Digital Features designed to increase the reach, readability, and engagement of peer-reviewed data.

VISUAL ABSTRACTS
Single-slide/graphical summaries optimized for social media.

VIDEO ABSTRACTS
Short videos where authors explain study background & significance.

PODCASTS
'Publication Pulses' – Audio summaries for multitasking physicians.

ANIMATED SUMMARIES
High-end 2D/3D animations explaining complex MOA.

INFOGRAPHICS
Long-form visual data stories for medical portals.
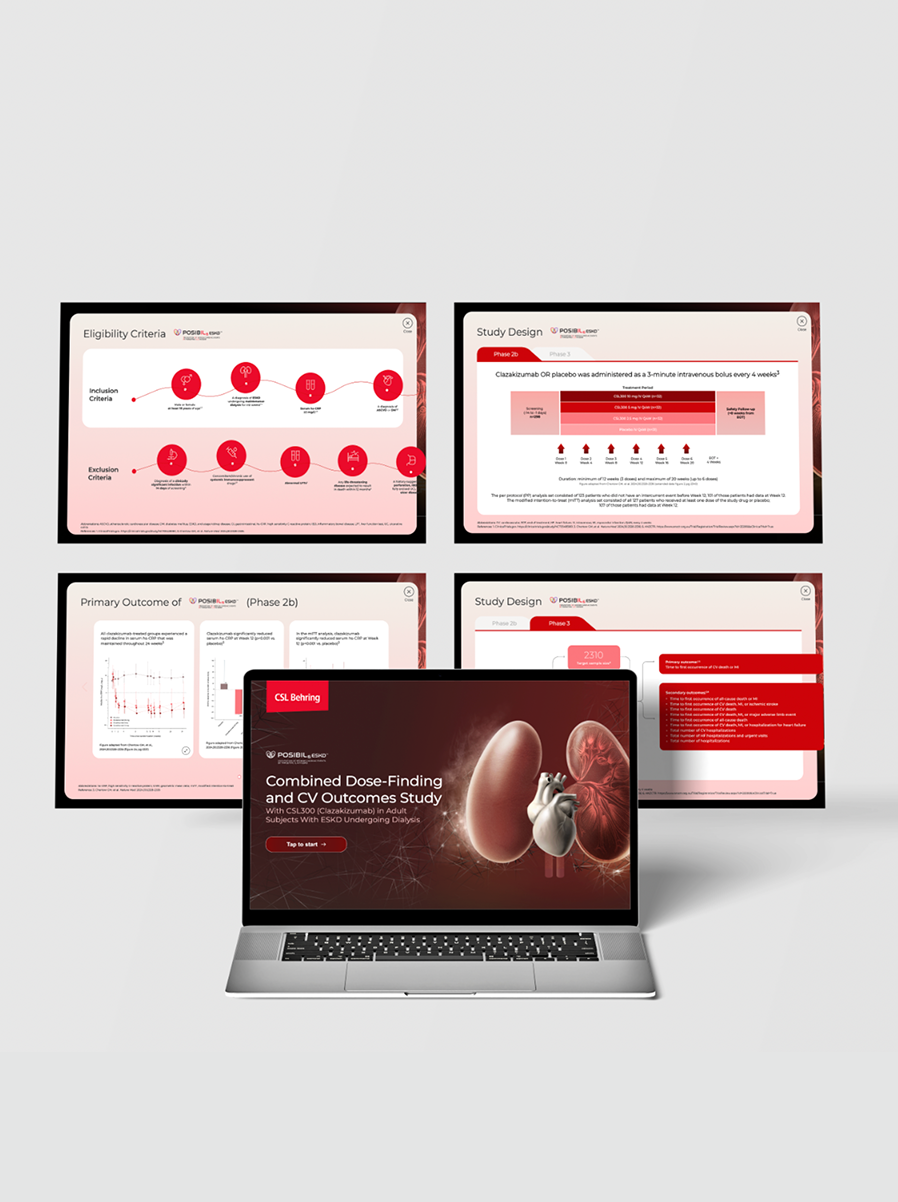
Healthcare Provider Communications
Healthcare Provider Communications
- Peer-reviewed journal article reprints
- CME program development
- Scientific symposia planning
Clinical Evidence
- Conference presentations & slide decks
- Poster and e-poster design
Digital Engagement
- Virtual symposia & webinars
- Digital learning platforms

Patient Education & Engagement
- Patient education brochures & disease awareness
- Patient decision aids & shared decision-making tools
- Multimedia content (video, animation, interactive)
- Patient mobile apps & digital health tools
- Social media strategy & content development